OMEROS CORPORATION: FDA Confirms Omeros' Schedule for Rolling Review of the Company's BLA for Narsoplimab in the Treatment of HSCT-TMA | FDA Health News

Frontiers | A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges With Current Regulatory Expectations

More Ice in the Winter Time: FDA Breakthrough Therapy Designations – Great PR While Patients Suffer — Innovation Breakdown: How the FDA and Wall Street Cripple Medical Advances by Joseph V. Gulfo
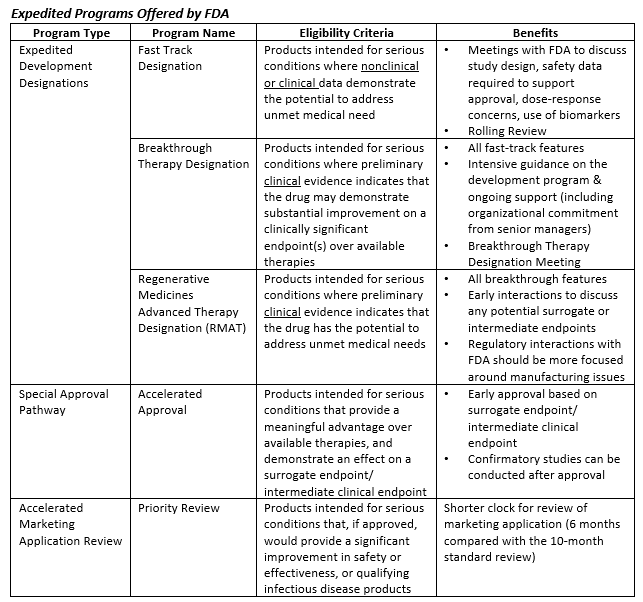
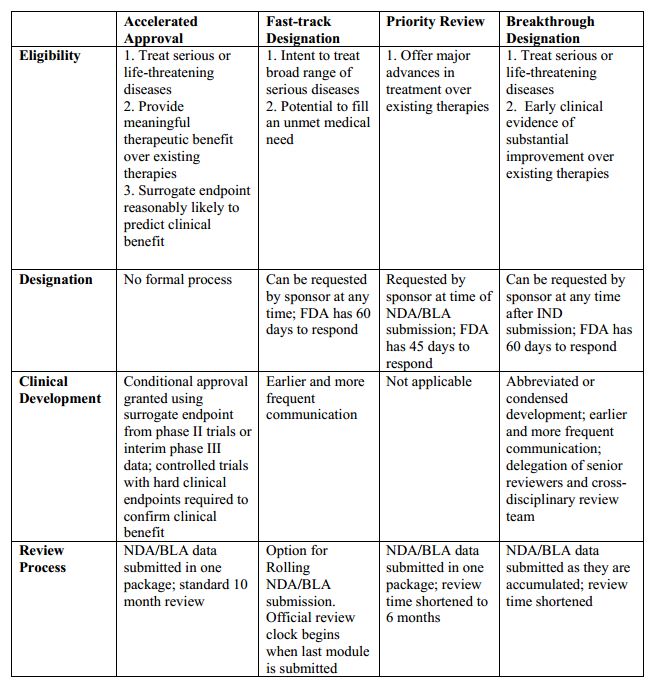
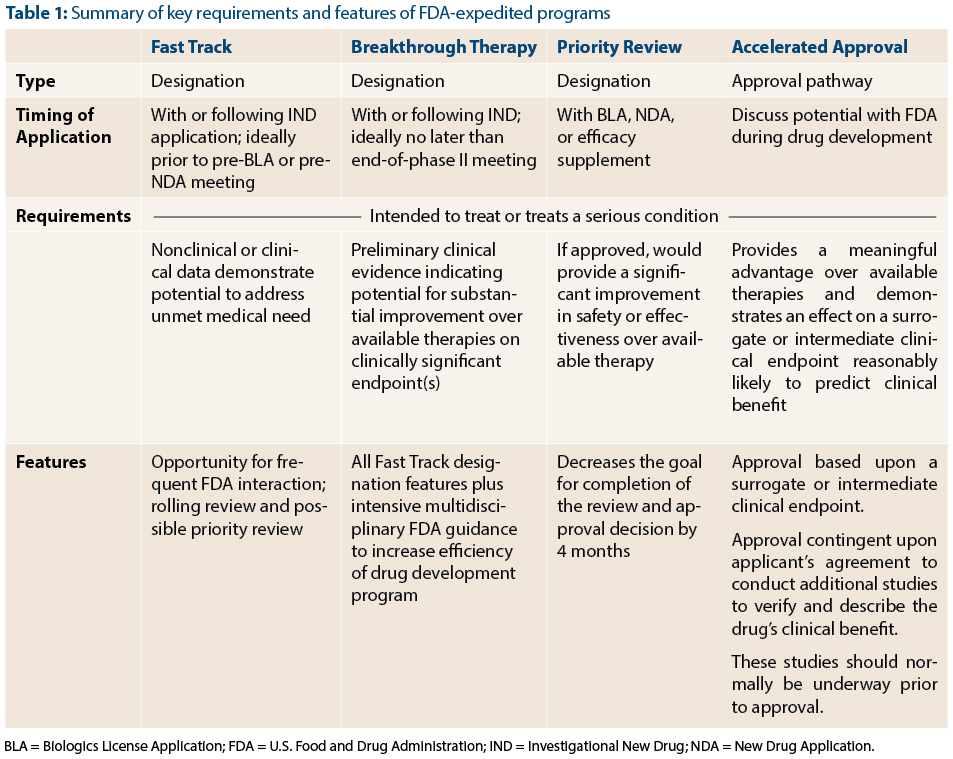
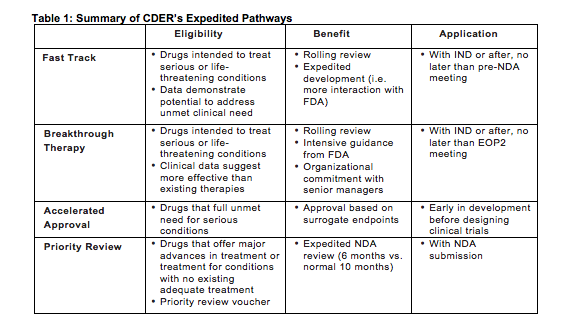
The Need for Speed in Drug Development: A Sponsor's Guide to FDA Expedited Programs | Halloran Consulting Group
![PDF] Expediting drug development--the FDA's new "breakthrough therapy" designation. | Semantic Scholar PDF] Expediting drug development--the FDA's new "breakthrough therapy" designation. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7a27650b2c8093898b53aeb13b50266c96048cae/3-Table2-1.png)
PDF] Expediting drug development--the FDA's new "breakthrough therapy" designation. | Semantic Scholar

CorMedix eyes US FDA priority review for bloodstream infection drug Defencath | S&P Global Market Intelligence
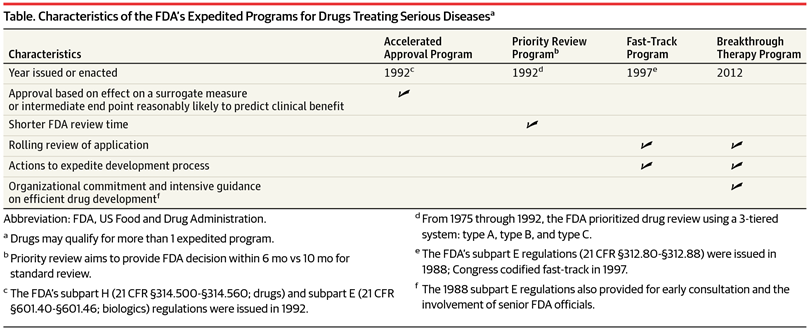
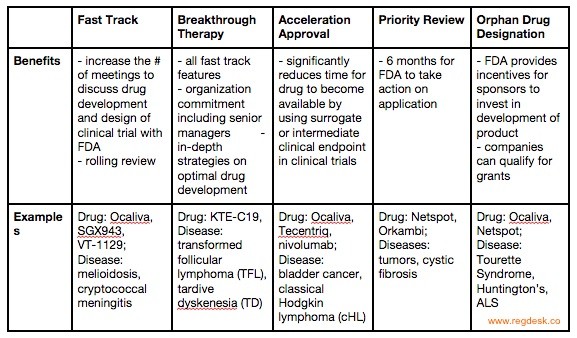
The Science Of A Biotech Valuation: How To Interpret The Value Of FDA Expedited Programs (NASDAQ:IBB) | Seeking Alpha

Nassau EM - What is the difference between an Emergency Use Authorization (EUA) and the Food & Drug Administration's normal medication/vaccine approval process? Before any medication can be used or prescribed in
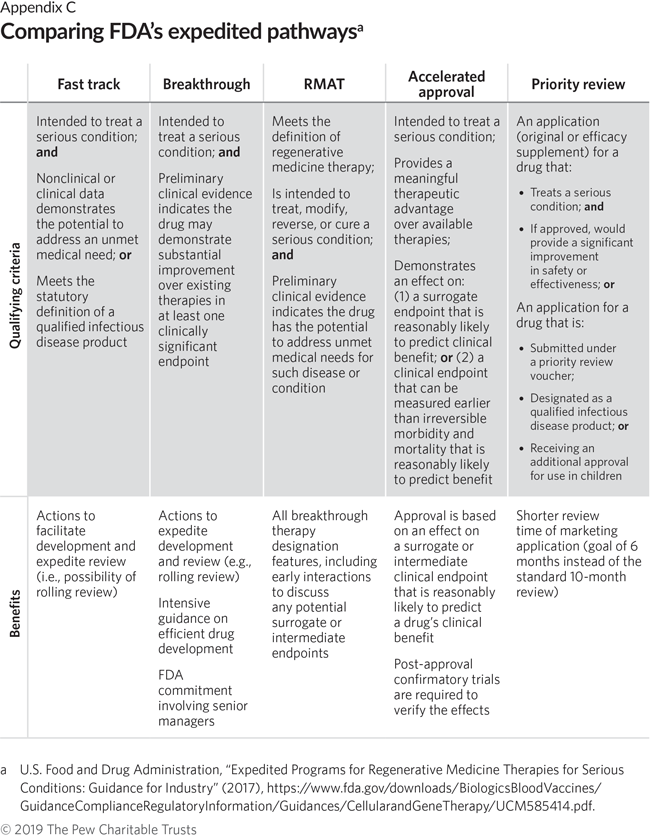
FDA's Framework for Regulating Regenerative Medicine Will Improve Oversight | The Pew Charitable Trusts